Abstract

Background: The presence of measurable residual disease (MRD) after achievement of remission with induction therapy is a prognostic marker of relapse risk in patients with acute myeloid leukemia (AML). Gilteritinib is an oral FLT3 inhibitor approved as a single agent for the treatment of patients with FLT3-mutated (FLT3mut+) relapsed or refractory AML. Evaluation of gilteritinib in the front-line setting is under way. We evaluated FLT3 internal tandem duplication (FLT3-ITD) mutation clearance using two different thresholds and correlated mutation clearance with survival outcomes in patients with newly diagnosed AML ineligible for intensive chemotherapy who were treated with front-line gilteritinib plus azacitidine (AZA) or either agent alone in the phase 2/3 LACEWING trial.
Methods: Adult patients with newly diagnosed FLT3mut+ AML ineligible for intensive induction chemotherapy received 28-day cycles of once-daily gilteritinib plus AZA in the Safety Cohort (80 or 120 mg/day gilteritinib plus 75 mg/m 2 AZA, Days 1-7) and in Arm AC (120 mg/day gilteritinib plus 75 mg/m 2 AZA, Days 1-7), gilteritinib (120 mg/day) alone in Arm A, or AZA (75 mg/m 2, Days 1-7) alone in Arm C. A subset of patients who had a best overall response of composite complete remission (CRc; defined as the sum of patients who achieved complete remission with or without complete hematologic or platelet recovery) and who had bone marrow-derived DNA samples available at baseline and at least one additional post-baseline timepoint were assessed for FLT3-ITD mutation clearance using next-generation sequencing. An Illumina ® sequencing platform was used to quantify FLT3-ITD and total FLT3 alleles. The FLT3-ITD variant allelic frequency (VAF) was defined as the ratio of FLT3-ITD to total FLT3 frequency. Data were analyzed using two different mutation clearance thresholds, FLT3-ITD VAF <10 −4 or <10 −3, where 10 −4 was based on previously published findings in patients with relapsed or refractory FLT3mut+ AML who were treated with gilteritinib (Altman JK et al., Cancer Med. 2021;10[3]:797-805) and 10 -3 was an additional exploratory threshold used because it provided a more balanced distribution of patients, given the small number of patients achieving mutation clearance at the 10 -4 threshold.
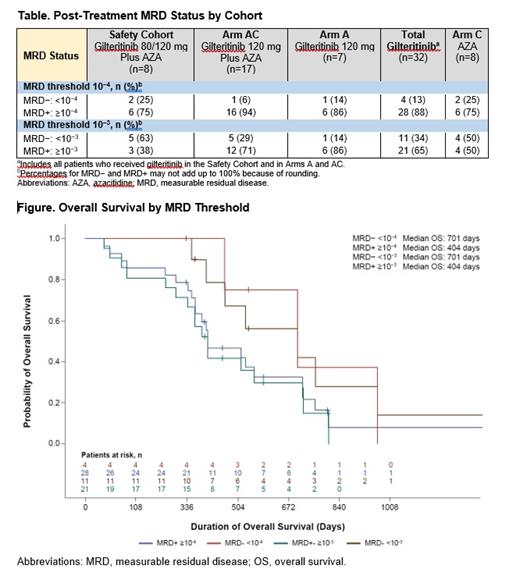
Results: The median age of patients enrolled in LACEWING was 77 years (range, 59-90), with 73% of patients aged >75 years. Although baseline characteristics of the overall LACEWING population were generally well balanced across treatment arms, higher proportions of patients treated with gilteritinib plus AZA (47%) or gilteritinib alone (59%) had an Eastern Cooperative Oncology Group (ECOG) performance status of ≥2 compared with patients treated with AZA alone (33%). Overall, 40 patients who achieved CRc and had sufficient DNA samples from bone marrow aspirates obtained at baseline and at least one additional post-baseline timepoint were included in the analysis (Safety Cohort, n=8; Arm A, n=7; Arm AC, n=17; and Arm C, n=8). Across both thresholds, the proportions of patients with FLT3 mutation clearance did not markedly differ between patients treated with gilteritinib or AZA (Table). In patients who received gilteritinib, FLT3-ITD mutation clearance using either threshold was associated with a similar increase in median overall survival (OS) compared to patients who did not achieve mutation clearance (Figure).
Conclusions: Regardless of MRD threshold, rates of MRD negativity were not substantially different between newly diagnosed FLT3mut+ AML patients ineligible for intensive induction chemotherapy who received gilteritinib alone, gilteritinib plus AZA, or AZA alone. Advanced age coupled with a worse baseline ECOG performance score at baseline may have compromised treatment response and achievement of FLT3 mutation clearance in patients treated with gilteritinib. The mutation clearance thresholds used in this analysis showed similar median OS in patients who received gilteritinib.
Wang: Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria, Other: Advisory Board; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Takeda: Consultancy, Honoraria, Other: Advisory board; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Mana Therapeutics: Consultancy, Honoraria; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Speakers Bureau; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy. Altman: Kartos: Research Funding; Theradex: Consultancy, Other: Advisory boards; Biosight: Consultancy, Other: Travel fees, Research Funding; Daiichi Sankyo: Consultancy; AbbVie: Consultancy, Other: Advisory Board, Research Funding; BMS: Research Funding; Amgen: Research Funding; Astellas: Consultancy, Other: Advisory Board, Research Funding; Fujifilm: Research Funding; ALZ Oncology: Research Funding; Immunogen: Research Funding; GlycoMimetics: Other: Participation on an advisory board; Syros: Consultancy; Kura Oncology: Consultancy; Boehringer Ingelheim: Research Funding; Aprea: Research Funding; Kura: Research Funding. Minden: Astellas: Consultancy. Wu: Astellas: Current Employment. Rich: Astellas Pharma Global Development, Inc.: Current Employment. Hill: Ligacept, LLC: Current holder of individual stocks in a privately-held company, Other: Stockholder; Astellas Pharma Global Development: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal